What Is Menopause?
Table of Contents

What is menopause?
You’ve heard of it, you even think you might be close to it, but what exactly is menopause?
Menopause is a universal female experience and refers to the time when a woman’s reproductive years come to an end. And it’s estimated that 25 million women globally go through it each year. Menopause begins with perimenopause and culminates when ladies enter a new stage in their lives as their periods end. This is known as post-menopause. For this reason, it’s often called ‘the change’ or the ‘change of life’.
In popular culture, the subject of menopause is still relatively taboo. Therefore, women can find themselves completely blindsided by the arrival of perimenopause. Until it rears its head they may have only held – perhaps – a vague awareness to expect a few hot flushes in the distant future. But there are 34 recognised symptoms of perimenopause we should be aware of as we move into our 40s.
So let us take you by our modern hand (read: we talk about menopause!) and give you back your power while we guide you through what you need to know.
The definition of menopause
The term menopause comes from the Greek language. Indeed, they give us the words ‘pausis’ (pause) and mēn (‘month’). Literally, menopause means the last period.
Do you remember puberty? Although menopause is often referred to as puberty in reverse you were probably more well-informed about what to expect back then. There’s a huge lack of education around menopause. Huge.
Menopause is defined as having occurred when a woman has not had her menstrual period for 12 consecutive months. And this typically occurs around the age of 51/52.
However, the term you should be most aware of is ‘perimenopause’ or the lead up to menopause. Indeed, this is often a time of hormonal chaos and when most symptoms occur.
Confusion reigns because the word ‘menopause’ is commonly used when referring to perimenopause. The truth is a woman will only know she has reached menopause itself in hindsight by which time she is already post-menopause.
NB: Menopause may occur early for you if you have:
- a family history of early menopause
- had a full hysterectomy
- been through cancer treatment
- smoked
- Some research suggests weight may contribute
What happens during menopause?
It’s more accurate to ask what happens during perimenopause as this is when many of the hormonal changes begin.
In simple terms, a woman’s ovaries gradually begin to produce less and less of the female hormone estrogen until she stops producing eggs. As a result, this end-point signals the body’s natural evolution from child-bearing years to non-fertile years.
Not all estrogen is the same
Technically speaking, the term estrogen refers to a group of hormones. During our reproductive years, estradiol (E2) is abundant and decreases at perimenopause. And estrone (E1) is the type of estrogen most dominant in post-menopause. (Estriol (E3) is present during pregnancy.)
As a result of the decline of the ovarian hormones at perimenopause, the brain may detect lower levels of E2. So the brain tries to produces more to encourage fertility which can lead to estrogenic fluctuations.
This is why perimenopause can be tough for approximately 70 per cent of women. The fluctuations can lead to hot flushes, rage, mood swings, sleep disturbances, depression, anxiety and insomnia. But those are just a few of the signs of menopause. There are several others to be aware of.
And what many women don’t realise is that there are estrogen receptors throughout the body and E2 plays a major role with many of our organs, not just our ovaries. Consequently, as E2 supplies diminish we become more susceptible to high cholesterol, heart disease, blood pressure, blood sugar imbalance, osteoporosis and changes in brain cognition. In addition, thyroid dysfunction and arthritis/rheumatoid arthritis can also occur at this time.
The stages of menopause
- Perimenopause – the years preceding menopause itself when most symptoms arise.
- Menopause – by definition menopause occurs 12-months after our last period.
- Post-menopause – fertility and menstruation have finished.
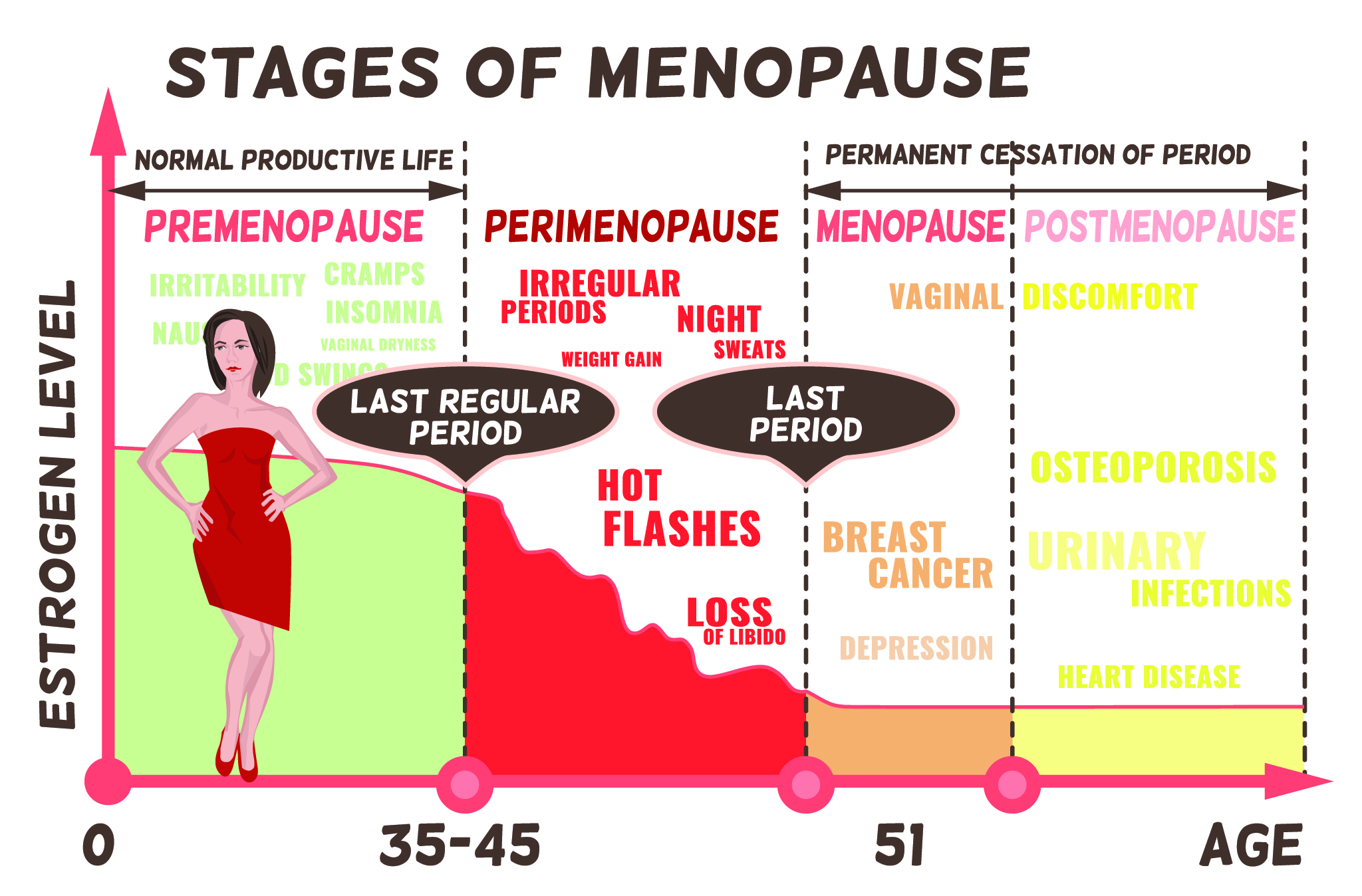
Perimenopause
Peri = ‘near’
Most females begin to experience the symptoms of perimenopause in their mid-forties. Although your progesterone levels decline from your mid-30s, it’s generally from around 40 that the rest of your sex hormones begin to follow suit.
Perimenopause is a different experience for every woman and some women may barely notice it. The first indicators are usually changes to the monthly cycle. And for some ladies, this can be accompanied by things like sore breasts, mood swings, weight gain around the belly, and fatigue.
For those with symptoms it can be a challenging time physically, mentally and emotionally.
Importantly, perimenopause lasts – on average – four to 10 years. The transition is usually a gradual process and many women enter perimenopause without realising.
Menopause
Menopause itself is something of a non-event. Even though you’ve likely stopped ovulating years before, biologically speaking menopause occurs after our last period. And it’s something you only know you’ve gone through when a full year has passed.
NB: If 12-months have passed without a period and bleeding begins again see your doctor.
Post-menopause
This is the time when menstruation is well and truly over. Indeed, the ovaries have stopped producing high levels of sex hormones and for many women, perimenopause symptoms subside.
E2 has protective qualities and diminished levels mean organs such as your brain, heart and bones become more vulnerable. Moreover, it’s a key lubricant so your lips may become drier, your joints less supple and your vagina might be drier. In addition, your thyroid, digestion, insulin, cortisol and weight may alter.
At this juncture, a woman might experience an increase in the signs of reduced E2 but she should have a decrease of perimenopause symptoms. That said, some women will experience symptoms like hot flushes for years or even the rest of their lives.
How long will the menopausal years last?
Two years? Five years? 10 years? Nobody can tell you how long your menopausal years will last. There’s a lot of conflicting information out there and there are always people who are exceptions to the rule.
On average, perimenopause begins four years before your last period. But in some cases, it can be 8-10 years beforehand.
Generally, females stop menstruating in their early fifties but everyone is an individual. So some women will experience this earlier, others later.
But the general consensus is four-10 years. Although renowned women’s health expert and the author of The Wisdom of Menopause Dr Christiane Northrup OB/GYN advises we use a guideline of 6-13 years.
What to expect during menopause
Fact. Half of the population is going to go through menopause. However, there’s a stigma attached to it in the Western world. Maybe it’s due to the stereotypical image of a frumpy, middle-aged woman with curlers in her hair or something similar. Alternatively, association may also be part of the reason. Not too long ago doctors diagnosed women of a certain age experiencing symptoms with ‘hysteria’ and sent them away to sanitariums.
We prefer the ancient wisdom of the Asian world where Traditional Chinese Medicine (TCM) refers to menopause as the ‘second spring’ or a time of wisdom and creativity. And Ayurveda looks at the life stages of women as maiden, mother and crone (wise one).
Thankfully, these days the old-fashioned stereotype is being blown to smithereens by females themselves. Indeed, they present as successful, radiant, vibrant, well-cared for and are flying the flag of empowerment. Think of Michelle Obama, J K Rowling and Oprah. Then there’s Gwyneth Paltrow who famously brought perimenopause out of the closet when she shared she was going through it on GOOP. Additionally, Angelina Jolie publicly highlighted surgically induced menopause when she underwent surgery to remove her ovaries at the age of 39.
What to expect emotionally
If you’re lucky you’ll experience nary a blip but if you’re like many of us you may find yourself on an emotional rollercoaster. As a result, you might lash out at loved ones, cry at the drop of a hat or want to stay in bed and hide under the covers. Other less talked about experiences can include a loss of confidence, anxiety and panic attacks at the thought of social gatherings and a feeling of becoming unhinged. These are typical perimenopause symptoms.
However, things can get more serious and this study showed there is a high rate of suicide among women aged 45-54 years which researchers found may be related to the biological changes of menopause. Consequently, if you are experiencing deep depression it can be a heavy load to bear. It’s important to reach out for help to people or organisations (like us) who understand. We can both support you and give you tools to get you through and find your mojo again.
Symptoms of perimenopausal depression:
- Low energy
- Paranoid thinking
- Irritability or hostility
- Decreased self-esteem
- Isolation
- Anxiety
- Somatic symptoms
- Sleep disturbance
- Weight gain
- Decreased sexual interest
- Problems with memory and concentration
What to expect physically
Other perimenopause symptoms include almost universally – highly symptomatic journey or not – periods becoming irregular. They can become shorter or longer and some women might live through inconvenient and distressing ‘flooding’.
Hot flushes are probably one of the most well-known symptoms but night sweats, sleeping difficulties, rapid heartbeat, headaches, gum problems, aching joints and bones, itchy skin, paresthesia, formication, weight gain, brittle nails, thinning hair, vaginal dryness, incontinence, bladder infections and painful sex can be commonplace too.
What to expect mentally
It’s not unusual to think you’re going crazy as you head into perimenopause. Particularly if you don’t realise you’ve reached this stage. Fortunately, you almost certainly haven’t lost your marbles but your brain is going through changes. Estrogen is an important part of brain function.
There are numerous estrogenic receptors in the brain and these help stimulate the ‘happy’ neurotransmitters serotonin and dopamine. They also promote brain energy and work with the hypothalamus (body temperature), the brain stem (sleep) amygdala (emotions) and hippocampus (memory). Dr Lisa Misconi, who leads the Women’s Brain Initiative research program at Weill Cornell Medical points to the brain first when talking about hot flushes, night sweats, insomnia, depression and anxiety.
“Those are neurological symptoms, we’re just not used to thinking of them as such,” she says. Many women can – and do – confuse symptoms like brain fog and forgetfulness with dementia but it’s often purely menopause-related. However, females are more affected by Alzheimer’s and dementia than males so it’s a good idea to seek medical advice.
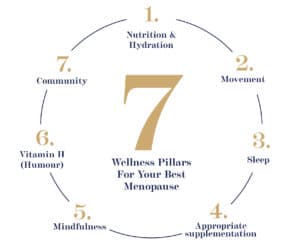
Relief through menopause
Knowledge is power. And when it comes to this menopausal transition nothing more could be truer than true (thank you, Dr Seuss!)
Diet
What you eat is vital to your menopausal journey. Typically a Mediterranean-style diet abundant in fresh fruit and vegetables accompanied by good quality lean proteins and healthy fats has been found to help manage symptoms.
Kicking processed foods, sugar, refined carbohydrates, caffeine and alcohol to the curb is also imperative as these have been linked to sleep disruption, hot flushes, mood swings and weight gain. Sugar and refined carbohydrates (which convert to sugar) cause blood sugar imbalances and insulin resistance which encourages the body to store fat.
Note: We’re referring to refined carbohydrates. Carbohydrates are macronutrients and along with the two other key macronutrients protein and fats are essential to health. Whole food carbohydrates such as vegetables, brown rice, quinoa and oats are good choices and help to keep your blood sugar balanced.
Use PPFF as a guideline: Phytoestrogens, Protein, Fat and Fibre
Phytoestrogens
Phytoestrogens are naturally occurring estrogens similar in structure to our body’s natural estrogen. They’re abundant in plant foods, seeds, legumes, healthy oils and soy foods (edamame beans, tempeh, miso or tofu) and can help with low estrogen and improve menopausal symptoms in some women.
NB: there is some controversy regarding soy studies particularly to do with breast cancer. Harvard gives a good common sense overview here.

Protein
Protein provides us with 20 amino acids, nine of which are essential but the body doesn’t produce them. This is why it’s important we get them from our diet. The building block of hormones, tissues and muscle, protein keeps us fuller for longer and stabilises our blood sugar reducing menopausal cravings for sugar and refined carbs. Women in perimenopause and beyond often struggle with this and experience sudden weight gain as a result. Protein has been shown to be beneficial for weight loss and maintenance.
The Ministry of Health for Australia and New Zealand recommends women aged 19-70 eat 46 grams of protein per day. Food provides us with either complete or incomplete proteins. Complete proteins provide the nine amino acids required from the diet, incomplete proteins require us to eat a variety of proteins to get all nine.
Meat as a source protein
Meat is a complete protein but it can be difficult to digest as we grow older. Further, there are a lot of studies linking it – in some cases tripling the risk – of cardiovascular disease. Quinoa, chia seeds and soybeans (tofu and tempeh) are plant-based complete proteins. Oats and green powders like spirulina and chlorella and nuts and seeds are also high in protein.
Eggs as a source protein
Eggs and dairy products like milk, yoghurt, cheese and kefir are also good sources of protein. In addition, they have the added benefit of providing calcium which is essential for bone density. This is important during the menopausal years as we become at risk of osteoporosis. Tip: the majority of people automatically think of milk and dairy as places to get calcium. But one of the best and more easily digested sources are dark leafy greens like spinach and kale.
Healthy fats
Fat is fabulous for satiety (feeling full) and keeping blood sugar balanced. Healthy fats refer to oily fish like salmon and sardines, avocado, walnuts, almonds, hemp seed oil, extra virgin olive oil, coconut oil and chia, sunflower, sesame and flaxseeds. Most of these contain the essential fatty acids omega-3 and omega-6. These are called essential as our body doesn’t make them so we rely on food to provide them.
Among their many benefits, healthy fats are linked with a lower risk of two things women become vulnerable to as we grow older – heart disease and breast cancer.

Fibre
Eating plenty of the fruits, vegetables and complex carbohydrates we’ve already noted is going to cover off your dietary fibre needs beautifully. Dietary fibre is the indigestible part of fruit, vegetables, whole grains like oats, and quinoa and legumes. There are two types of fibre: soluble and insoluble. Soluble fibre helps keep blood sugar nice and balanced, aids good digestion and keeps you fuller for longer. Insoluble fibre soaks up water, softening your poop and cleaning out your colon.
Exercise
It’s important to move your body at least 30 minutes per day for a gamut of reasons from mental health to bone density. Many women also embrace it for weight management and it certainly helps to keep your metabolic rate at a good level. However, it’s important to understand that weight gain and loss is 80 per cent to do with what you eat.
Anyone who knows the wonders of exercise knows it can deliver an instant feel-good factor. That’s the endorphins, neurochemicals the body releases as we exercise that trigger a feeling of wellbeing.
Two main forms of exercise
There are two main forms of exercise: cardiovascular and resistance training. Cardiovascular refers to any exercise that raises your heart rate like walking, running, cycling, swimming, jumping rope and HIIT. Weight training uses resistance to strengthen muscles. This could be dumbbells, weight machines, your own body weight (as in Yoga) or water bottles.
Both forms are crucial during menopause for overall wellness, weight management, and mental health. Resistance training also increases bone density so when we consider the high risk of weakened bones and osteoporosis making it part of a weekly schedule is a no-brainer.
More and more studies are pointing to HIIT (High-Intensity Interval Training) as beneficial for women aged over 40. Especially when it comes to weight management, managing inflammation and bone mineral density. HIIT alternates short bursts of intense aerobic exercise with intervals of more moderate movement.
The bonus for women experiencing some of the more common symptoms of peri/menopause? Exercise endorphins help reduce stress, anxiety, depression, boost self-esteem and curb appetite. And all that energy burned? It just might – and almost definitely will – improve your sleep.
NB: One caveat is not to overdo it or go too hard out as this can stress your body and put the adrenals into overdrive.
Supplements
The transition to our ‘second spring’ (don’t you love that term?) sees our bodies going through a lot of change and some of it is stressful. Indeed, this is a time when we become far more vulnerable to nervous tension so it’s crucial we find ways to manage this. And stress depletes estrogen even more.
You see, the adrenals (tiny glands that produce the stress hormone cortisol) take over from the ovaries in producing some estrogen. And when we’re stressed cortisol takes priority. So when cortisol escalates, estrogen declines.
This could lead to burning through more nutrients because adrenalin/cortisol release is all about short term survival, or stress-related digestive issues may inhibit optimum nutrient absorption. Nutrients are vital when transitioning into menopause. A deficiency in some of them could well be behind mood swings, depression and some of the aches and pains you may be putting down to perimenopausal symptoms. Indeed, this study specifically looked at the vitamin B group, vitamin C, vitamin D, and vitamin E and their impact of health and quality of life during menopause.
Some recommended supplements include*:
*This is not a fully comprehensive list, nor does it cover the entire scope of benefits. If you would like more information please visit the Ministry Of Health for Australia and New Zealand, US National Institutes of Health fact sheets or Harvard Health’s listing of vitamins.
- A good quality multivitamin and mineral supplement.
- Adaptogens such as ashwagandha and rhodiola can stabilise the adrenals and the stress hormone cortisol.
- Calcium helps with bone density. It’s the most abundant mineral in the human body and has been linked to the prevention of fractures, osteoporosis and diabetes.
- Essential fatty acids (EFAs). Omega 6 (linolenic acid) and Omega 3 (alpha-linolenic acid) are polyunsaturated fats known as essential fatty acids because our bodies can’t produce them. The ideal ratio for wellness is 3:1 however many western diets are too heavy on omega 6 at 20:1. EFAs help with hormone balance, mental health, heart and liver health, weight management and inflammation. The family also include omega 9 which is a monounsaturated non-essential fat.
- Iron is an essential mineral that transports oxygen throughout the body. A deficiency can lead to anaemia and it’s one of the most common deficits in women. Levels could also be vulnerable during perimenopause when experiencing heavy bleeding.
- Magnesium partners with calcium and helps to transport it to where it needs to go. Low levels have been linked with low bone mass. An essential mineral that can be lacking in our diets, magnesium is calming and has been found to be useful for anxiety, sugar cravings, muscle cramping and migraines.
- Probiotics for gut health. If you’re having digestive issues (or would like to ward them off) a probiotic can be helpful. Alternatively, eat fermented foods like sauerkraut and kimchi.
- (The) B vitamins
The vitamin B family includes B1 (thiamine), B (riboflavin), B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folate) and B12 (cobalamin). Of these, the body can only store B12. Together they B’s are necessary for overall health and wellbeing including heart, brain, metabolism, immunity, inflammatory conditions, cognition and liver detoxification. We’re calling out B6 and B12 for the menopausal journey as vitamin B6 has been linked with releasing the neurotransmitters serotonin and dopamine which helps with mood and may even diminish panic attacks, and vitamin B12 deficiency has been linked with dementia and depression. A high-quality vitamin B complex is a good tool and we highly recommend it. - Vitamin C is an interesting one. Most of us associate with immunity and it definitely plays a major role there. It has other key functions too including the production of collagen for our joints and skin, oral health and bone health. It’s also a natural antihistamine so if you’re suffering from itchy skin, paresthesia or formication vitamin C supplementation and/or topical applications using it could be helpful. The body can’t store vitamin C so we need to eat fruit and vegetables regularly to get our quota. This study showed vitamin C could also be useful for anxiety and stress-related high blood pressure.
- Vitamin D is considered essential because it supports the absorption of calcium in our bodies helping skeletal strength and muscle function. Also known as the sunshine vitamin we naturally make vitamin D with exposure to sunlight. A deficiency increases our risk of osteoporosis, bone fractures and bone pain. The recommended variant is vitamin D3 – the same form as the body makes from sunlight.
- Vitamin E is a powerhouse of an antioxidant and helps reduce stress, inflammation in the body, depression, heart disease. Oh, and our skin loves it. Studies have shown it could provide a viable alternative to estrogen treatment for vaginal dryness/burning.
- Zinc is an essential trace element that’s used to create hormones and also balance them. It can help balance the thyroid and cortisol hormones, high blood pressure and blood sugar balance. This study shows its benefits when used in conjunction with other minerals like magnesium and copper for post-menopausal women with low bone density.
- 40+ from MenoMe® uses Estrog-100™, a herbal phytoestrogen blend of cynanchum wilfordii, phlomis umbrosa and angelica gigas nakai to balance estrogen. It has been clinically proven to help with 10 of the main symptoms of menopause.
- 55+ from MenoMe® uses Estrog-100™ as above with the addition of Enzogenol® pine bark extract, marine magnesium and vitamin D3.
Disclaimer: Our articles are for information only and intended to educate from a holistic point of view. They should not take the place of medical advice. Certain diet and exercise practise may be beneficial for the majority of people but could have potential risks for others. If you are contemplating lifestyle changes or you have ongoing concerns please consult your healthcare provider.